Part 2A: TESTING CATALASE ACTIVITY
Introduction: This part of the of the experiment is focused on how changing temperatures and the presence of living tissue will affect an enzyme’s ability to break down H202.
Procedure:
1. To observe the reaction to be studied, transfer 10-mL of 1.5% (0.44 M) H2O2 into a 50-mL glass beaker and add 1 mL of the freshly made catalase solution. The bubbles coming from the reaction mixture are O2, which results from the breakdown of H2O2 by catalase. Be sure to keep the freshly made catalase solution on ice at all times.
2. To demonstrate the effect of boiling on an enzymatic activity, transfer 5-mL of purified catalase extract to a test tube and place it in a boiling water bath for five minutes. Transfer 10-mL of 1.5% H2O2 into a 50-mL glass beaker and add 1-mL of the cooled, boiled catalase solution.
3. To demonstrate the presence of catalase in living tissue, cut 1 cm3 of potato or liver, macerate it, and transfer it into a 50-mL glass beaker containing 10-mL of 1.5% H2O2.
Discussion / Analysis:
The enzyme used in this experiment was the catalase, while the substrate was the H202. The first part of the experiment was used to measure enzyme activity when kept at a cold temperature. The second step of the procedures would have been used to measure enzymatic activity at hot temperatures. Lastly, the third part of the procedures would have been used to demonstrate the presence of catalase in living tissue. The results from this exercise could have been used to determine at what temperatures the enzyme works best. The temperature at which the enzyme works best would have the highest recorded results for presence of oxygen or bubbles when mixed, since these bubbles and oxygen indicated that H2O2 is being decomposed.
Part 2B: ESTABLISHING A BASELINE
Introduction: The baseline is done in order to determine the initial amount of H2O2 in a 1.5% solution. All the following steps involve no use of the catalase. The amount of H2O2, the baseline, is an index for the initial concentration of H2O2 in the solution. For any experiments conducted, it is important to first establish a baseline.
Procedure:
1. Put 10-mL of 1.5% H2O2 into a clean glass beaker.
2. Add 1-mL of H20 (instead of enzyme solution)
3. Add 10-mL of H2SO4 (1.0 M). *Use extreme care in handling reagents
4. Mix well.
5. Remove a 5-mL sample. Place this 5-mL sample into another beaker and assay for the amount of H2O2. Use a burette, a syringe, or a 5-mL pipette to add KMnO4, a drop at a time, to the solution until a persistent pink or brown color is obtained. Remember to gently swirl the solution after adding each drop.
Baseline Calculations:
Analysis:
It is important to first establish a baseline in order to have some type of group for comparision. Once the initial amount of H202 is found, it will be possible to compare and calculate what amount from the initial amount of H202 was dissolved by the enzyme catalyse. For our results, we found that there was an initial amount of 3.1 mL of H202 in the substrate. This amount will later be used as a basis for calculating how much H2O2 is decomposed in an uncatalyzed reaction. However, since more than a day will have passed since establishing this baseline, for exercise 2D, the baseline will have to be done again.
Part 2C: UNCATALYZED REACTION RATE OF H2O2 DECOMPOSITION
Introduction:
In this exercise, the objective is to measure the amount of H2O2 that is decomposed in an uncatalyzed reaction.
Procedure:
1. To determine the rate of spontaneous conversion of H2O2 to H2O and O2 in an uncatalyzed reaction, put a small quantity of 1.5% H2O2 (about 15-mL) in a beaker. Store it uncovered at room temperature for approximately 24 hours.
2. Repeat steps 2-5 from Part 2B to determine the proportional amount of H2O2 remaining. Record your readings in the box below.
1. Put 10-mL of 1.5% H2O2 into a clean glass beaker.
2. Add 1-mL of H20 (instead of enzyme solution)
3. Add 10-mL of H2SO4 (1.0 M). *Use extreme care in handling reagents
4. Mix well.
5. Remove a 5-mL sample.
Analysis/Conclusion:
Our results demonstrated that about 3.1 mL of KMnO4 of the titrant was used, based on the readings of the burette. For calculation purposes, it is assumed that 1 ml of KMnO4 is equivalent to 1 mL of H202 in the solution. Therefore, when the 3.1 mL of KMnO4 was subtracted from the initial amount of H2O2 found in the baseline, which was also 3.1 mL , the difference was zero. Such low results of H2O2 decomposed may have been due to human error, such as not cleaning out the beaker or incorrect measurements. There may have also been an error when establishing the baseline in part 2B. Yet, the results suggest that the decomposition of H2O2 occurs at a very slow pace without the catalyse.
Part 2D: CATALYZED REACTION RATE OF H2O2 DECOMPOSITION
Introduction:
The purpose of this exercise is to determine how much H2O2 is decomposed in an catalyzed reaction at different time intervals.
Procedure:
1. If a day or more has passed since you did Part 2B, you must reestablish the baseline by determining the amount of H2O2 present in your 1.5% solution.
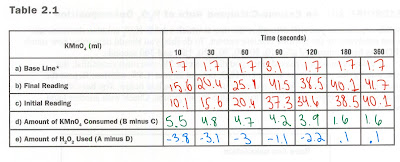
NEW Baseline Calculations:
2. Repeat the assay procedure (steps 1-5) and record your results in the box below. The base line assay should be approximately the same value for all groups.
1. Put 10-mL of 1.5% H2O2 into a clean glass beaker.
2. Add 1-mL of H20 (instead of enzyme solution)
3. Add 10-mL of H2SO4 (1.0 M). *Use extreme care in handling reagents
4. Mix well.
5. Remove a 5-mL sample.
Analysis/Conclusion:
Our results demonstrated that as more time progressed, the more H2O2 was decomposed. The value of H2O2 decomposed or used was calculated the same way as it was for Part 2C. Our results show that at 10 seconds a negative amount of H2O2 was used. Yet, when the substrate was allowed 360 seconds of time to react with the enzyme, there were .1 mL of H2O2 decomposed. The graph's negative slope shows a decrease in the amount of H2O2 present after longers period of time. This supports the idea that the more time that passes and the longer the enzyme is allowed to react, the more H2O2 that is decomposed. This is why there are lower levels of H2O2 over time. The majority of it is decomposed, and it becomes water and oxygen.



Enzyme catalysis is a procedure to increase the rate of virtually all the chemical reactions within cells by the active site of a protein. Enzyme may be part of a multi-subunit complex. It may also transiently or permanently conjugate with a cofactor. enzyme catalysis
ReplyDelete