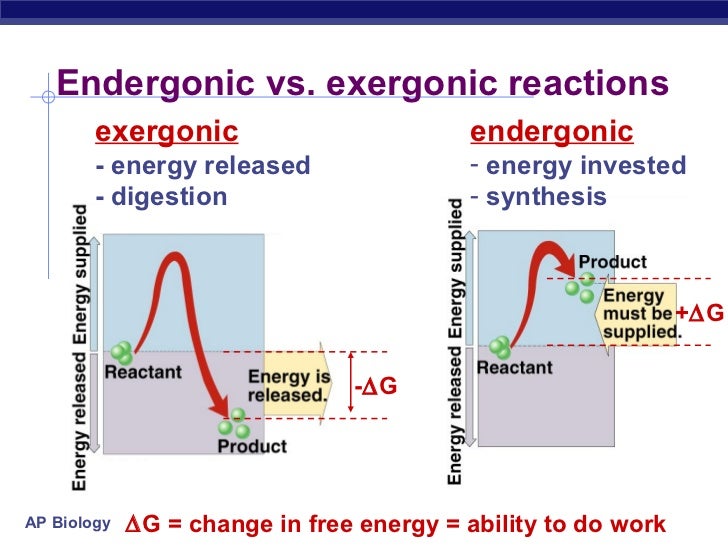
- Compare and contrast Endergonic and Exergonic reactions - include a model of each showing the change in free energy from reactants and products.
Endergonic type of reactions absorb energy. They are anabolic and non-spontaneous, meaning that they require energy input. They will also have a positive net change in free energy. Exergonic reactions will release energy into the surrounding area. They are catabolic and spontaneous, meaning that they do not require energy input. Their net change in free energy is negative. This free energy is able to perform work, as long as the temperature and pressure are maintained constant.
 |
| Source: www.slideshare.net |
- Explain the key role of ATP in energy coupling reactions. Provide a model showing energy before and after phosphorylation of the reactants.
One ATP, also known as Adenosine triphosphate, molecule is composed of:
- 1 Ribose, sugar, molecule
- 1 Adenine, nitrogenous base, molecule
- 3 Phosphate groups
Energy couple is when an exergonic reaction is used to drive an endergonic one. Through hydrolysis, one of the three phosphate groups will break off. The energy from this process comes from the change to a state of lower free energy. It DOES NOT come from the phosphate group breaking off. Phosphorylation is when the phosphate group that broke of the original ATP molecule binds to another new molecule, which is referred to as the phosphorylated intermediate. This then in a sense regenerates the ADP molecule into ATP.
| Source: cnx.org |
- Analyze data showing how changes in enzyme structure, substrate concentration, and environmental conditions (pH, temperature, salinity, etc.) affect enzymatic activity - ie. include a graph of each example and predict the effects when one of the parameters is further changed. Justify and explain your predictions.
Enzymes will function best only in certain conditions. Changes in the temperature and the pH of the substrate may cause the enzyme to become inefficient. The two graphs below demonstrate the effects of changes of pH on the enzymes pepsin and trypsin. The enzyme’s rate of reaction will peak only at either a pH level of 2 or a pH level of 8. As either the pHs stray from the optimal pH, whether there is an increase or a decrease in pH, the rate of reaction goes down.
The same thing is demonstrated in the second graph. The enzyme’s highest rate of reaction will only occur at a specific temperature.
 |
| Source: Pearson Education Inc. |
 |
| Source: Pearson Education Inc. |
- Describe the relationship between the structure and function of enzymes.
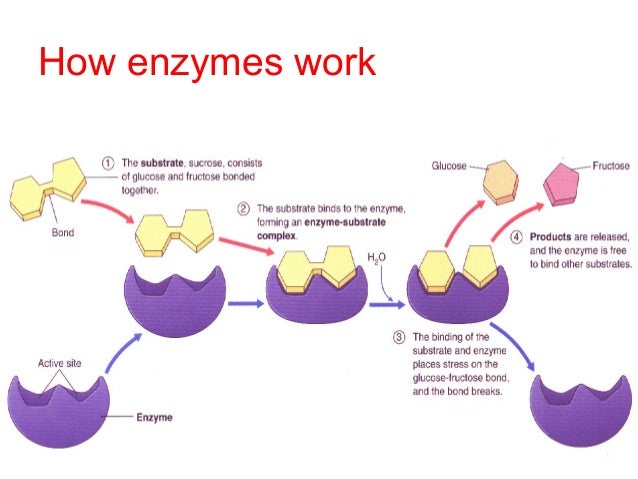
Enzymes are a special type of protein, relatively large in size, that will speed up a chemical reaction. They are able to do this without change to the amount of free energy available. This makes enzymes catalysts. The substance that a catalysts, in this case enzymes, acts on is referred to as a substrate. Only specific enzymes may catalyze certain molecules. What molecules the enzyme may act on is dependent on the enzyme's structure. The substrate must be able to bond to the enzyme's activation site and from the enzyme-substrate complex. It is only after this that the speeding up of the reaction occurs.
- Explain how enzymes accomplish biological catalysis. Provide examples.
Enzyme’s serve to speed up metabolic reactions without the use of free energy. Enzymes are able to achieve this by. however, by LOWERING the amount of free energy REQUIRED to make reactants into the necessary products. Without enzyme’s product production would become inefficient since the reactions would take too long.
 |
| Source: www.slideshare.net |
- Describe how enzyme-mediated reactions can be controlled through competitive and noncompetitive interactions.
Similar to the definition of inhibit, which is to hinder or prevent, and inhibitor will bond with an enzyme in order to stop its activity. Inhibitors can either be competitive or noncompetitive.
Competitive inhibitors will in a sense “compete” with the substrate. They will bind to the active site of an enzyme and compete with the substrate for the site and try to block the substrate from binding. On the other hand, non-competitive inhibitors will take a different route to stop the reaction and will instead bind to a different section of the enzyme. They will then change the shape of enzyme, thus making the active site less effective and stopping the reaction. Inhibitors control enzyme-mediated reactions by stopping them.
- Propose experimental designs by which the rate of enzyme function can be measured and studied.
A useful way to measure the functionality of an enzyme would be to measure the time it takes for the enzyme to actually complete the process, or in other words turn the substrate into products. This experiment would be similar to the one recently conducted in class. One would have to do several timed trials to see how much of the substrate reacts. Time would being when the catalyst is added to the substrate. The time would stop when the inhibitor is added. The, using a titration system, one would measure the amount of substrate still present or the amount that reacted, and thus measure the functionality of the enzyme. Enzymes that work better would cause more reaction in a shorter span of time.
No comments:
Post a Comment