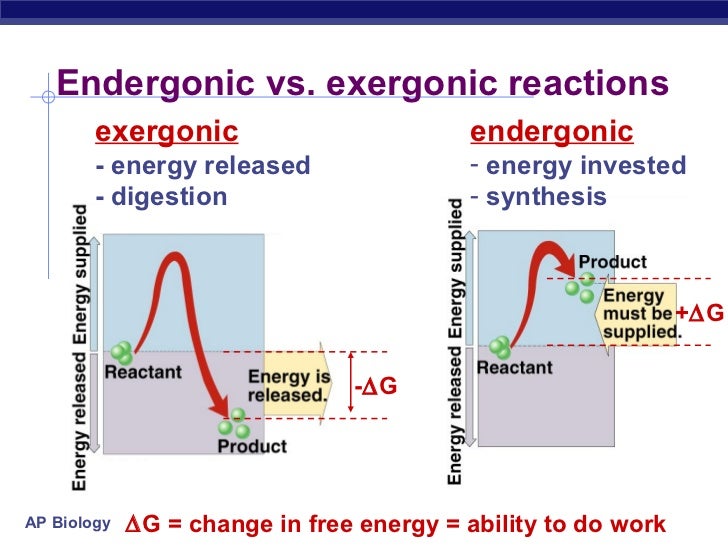
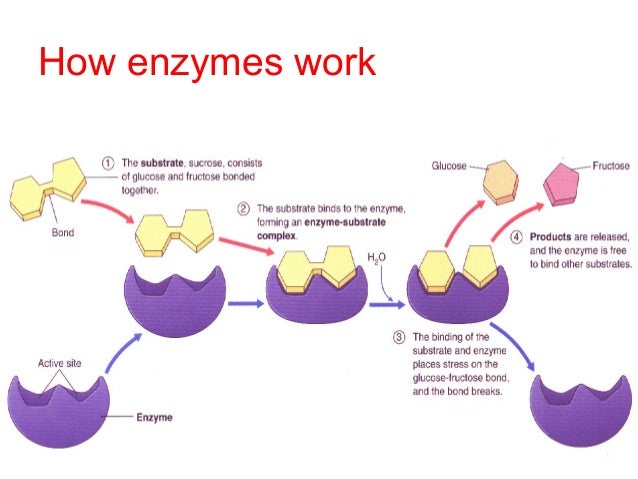
Passive transport is when materials are able to cross the cell membrane without the use of any energy in the form of ATP. Since the plasma membrane is selectively permeable, this means that only certain substances will be able to pass through. The membranes composition of fatty acid tails allows for nonpolar molecules to easily pass, without the use of a transport protein. It is through the process of diffusion that these molecules are able to do this.
SInce part of the plasma membrane is nonpolar, polar molecules will require special proteins that will carry them across the cell membrane when the cell needs them.
This protein is used to transport glucose molecules across the membrane. Located on the membrane of red blood cells, this protein will only accept glucose molecules to bring them into the cell. This type of transport DOES NOT require ATP.
Also known as the sodium-potassium pump, it is a type of active transport system, meaning that it does require ATP. This protein transport sodium and potassium against their concentration gradient. This means that it will take sodium from areas of low concentration and take them to areas of high concentrations. This helps the cell maintain concentration gradients that differ from their environment. It is like pushing a boulder up a hill instead of letting it roll down.
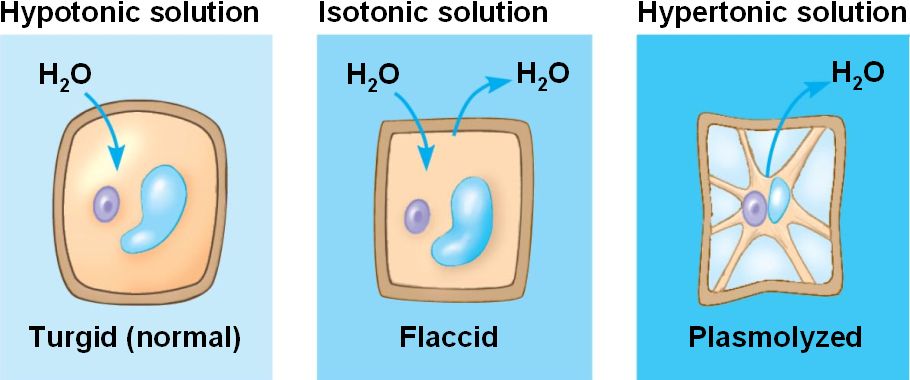
Hypotonic:
This is when there is less solute concentration inside of the cell. Water will rush INTO the cell. If immersed in a hypotonic environment, cell will SWELL and possibly burst (also known as a lysed cell) However, plant cells will not burst due to their cell walls. Is actually the best environment for plant cells.
Hypertonic:
This is when there is more solute concentration inside of the cell than there is outside. Water will rush OUT of the cell. If immersed in a hypertonic solution, the cell will shrivel.
Isotonic:
This is when the solute concentration is the same inside and outside of the cell. Animal cells, which do not have a cell wall will want to be in an isotonic environment.
Active transport is when ATP is necessary in order to get materials across the plasma membrane. This type of transport mainly takes place in the proteins that are embedded into the plasma membrane, including the sodium potassium pump. There also exists the electrogenic pump, which serves to transport protons across the membrane.
Endocytosis (Materials IN):
This process is used to bring materials INTO the cell. It may help to think of this process as “the cell eating,” also known as phagocytosis. The cell does this by creating vesicles from the plasma membrane to engulf particles from the extracellular fluid.
Exocytosis (Materials OUT):
The opposite of endocytosis, this process is when the cell transports materials out of it. It does this by creating transport vesicles inside of the cell. These vesicles will then travel to the membrane, fuse with it, and then release their contents. This is used to get rid of wastes and also export products.
Create a visual representation/model (ie. graph or diagram) to make predictions about the exchange of molecules between an organism and its environment, the use of these molecules (ie. CHNOPS and incorporation into carbohydrates, proteins, lipids, nucleic acids, membrane structure, genetic information, etc.), and consequences to the organism if these molecules cannot be obtained.
The interaction between plant cells and their exchange with water is dependent on the type of solution they are immersed in. Plant cells will desire to be in is hypotonic solution, so that water may rush into them and keep the cell walls rigid and stiff. Isotonic environments will be detrimental to plant cells since there is no water going in or out. Hypertonic environments are worse, causing water to leave the plant cells, making the plant limp and soggy.